Wnt signaling
Introduction.
The Wnt signaling pathway has been implicated in a wide range
of biological processes from maintaining the pluripotentiality
of stem cells to
the induction
of specific
tissues and organs during development. Moreover, a majority of the components
of the Wnt signaling pathway are found throughout the animal kingdom.
The Wnt signaling pathway
In the mouse there are 19 different Wnt genes encoding for
secreted proteins that signal by binding to their receptors.
The family of Wnt receptors is composed of 10 Frizzled genes.
Moreover, the co-receptors
LRP5 and LRP6 participate in the transmission of the Wnt signals. The Wnt
signals can be blocked by a series of secreted antagonists grouped
in two categories.
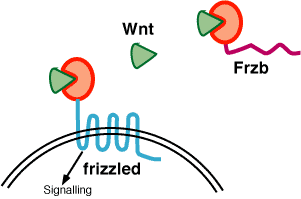 The
first group of antagonists is composed of Frzb and its four homologs,
which form the secreted Frizzled
Related Protein (Sfrp) family, as well as Wif and binds directly
to Wnt
proteins, thereby
blocking activation of the receptor (Leyns et al., 1997). Secreted
antagonists of the second category bind to the LRP co-receptors
thereby blocking the
activation of the Wnt signaling pathway. These antagonists
include Dkk1 and its three
homologs as well as Wise.
The
first group of antagonists is composed of Frzb and its four homologs,
which form the secreted Frizzled
Related Protein (Sfrp) family, as well as Wif and binds directly
to Wnt
proteins, thereby
blocking activation of the receptor (Leyns et al., 1997). Secreted
antagonists of the second category bind to the LRP co-receptors
thereby blocking the
activation of the Wnt signaling pathway. These antagonists
include Dkk1 and its three
homologs as well as Wise.
At the intracellular level, the activation of the Wnt receptors is
transduced by several pathways . The best characterized
one, called the canonical pathway, is based on a series of phosphorylation
steps that ultimately leads to the stabilization and nuclear import
of beta-Catenin. In the nucleus, beta-Catenin associates with TCF
factors
and this complex
activates target genes of the Wnt pathway. Several Wnts (Wnt1, -2,
-2b, -3, -3a, -6,
-7b,
-8a and -8b) have been shown to activate specifically the canonical
pathway.
The signaling by other Wnts (Wnt4, -5a and -11)
is mediated by non-canonical pathways involving intracellular
signaling
by Ca2+ or the JNK cascade.
The signaling pathways triggered by the remaining Wnts (Wnt5b, -7a,
-9a, -9b,
-10a, -10b and
-16) have not yet been characterized.
Due to technical limitations including those related to the
purification of Wnt proteins, the binding specificities of the
nineteen Wnt proteins
with their
ten
Frizzled receptors, and the eleven potential secreted antagonists, as
well as the integration of the signals through the various pathways,
have not
yet been
completely elucidated.
Current research
We
are currently investigating the binding specificities of several
Wnt proteins with secreted antagonists such as Frzb or with the
Frizzled receptors.
 Two complementary approaches are used. First, we measure the
biological activity of canonical Wnts such as Wnt3a by detecting
the stabilization of intra-cellular beta-catenin in cells treated
with Wnt. We then treated the cells with the Wnt protein as well
as with the secreted antoganist or a soluble version of the Frizzled
receptor. The binding of these proteins will reduce the amount
of free Wnt in the culture medium and therefore limit the extent
of beta-catenin stabilization.
Two complementary approaches are used. First, we measure the
biological activity of canonical Wnts such as Wnt3a by detecting
the stabilization of intra-cellular beta-catenin in cells treated
with Wnt. We then treated the cells with the Wnt protein as well
as with the secreted antoganist or a soluble version of the Frizzled
receptor. The binding of these proteins will reduce the amount
of free Wnt in the culture medium and therefore limit the extent
of beta-catenin stabilization.
With this approach we can analyse the binding specificities in
living cells.
The second approach is purely biochemical and involves measuring
the binding efficiency between purified Wnt proteins and sFRPs
or Frizzled using plasmon surface resonance (Biacore).
The combination of these two approaches with genes expression
studies showing when and where the extracellular members of the
Wnt cascade are expressed will allow us to refine our understanding
of the Wnt signals.
Up