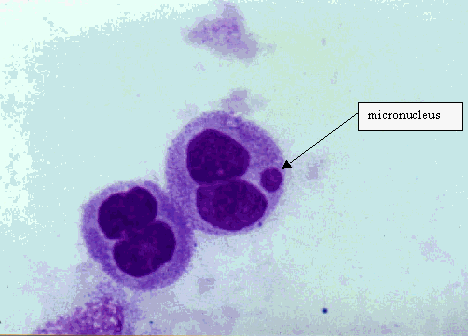
A micronucleus (MN) is formed during the metaphase/anaphase transition of mitosis (cell division). It may arise from a whole lagging chromosome (aneugenic event leading to chromosome loss) or an acentric chromosome fragment detaching from a chromosome after breakage (clastogenic event) which do not integrate in the daughter nuclei.
Scoring of micronuclei can be performed relatively easily and on different cell types relevant for human biomonitoring: lymphocytes, fibroblasts and exfoliated epithelial cells, without an extra in vitro cultivation step. MN observed in exfoliated cells are not induced when the cells are at the epithelial surface, but when they are in the basal layer.
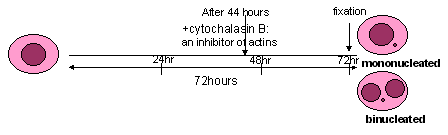
An ex vivo / in vitro analysis of lymphocytes in the presence of cytochalasin-B, an inhibitor of actins (added 44 hours after the start of cultivation), allows to distinguish easily between mononucleated cells which did not divide and binucleated cells which completed nuclear division during in vitro culture. Indeed, in these conditions the frequencies of mononucleated cells provide an indication of the background level of chromosome/genome mutations accumulated in vivo and the frequencies of binucleated cells with MN a measure of the damage accumulated before cultivation plus mutations expressed during the first in vitro mitosis.

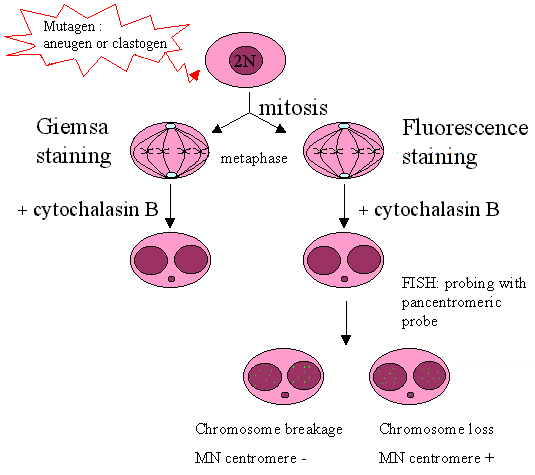
The combination of the micronucleus assay with fluorescence in situ hybridisation (FISH) with a probe labeling the (peri-)centromeric region of the chromosomes (FISH assay) allows discrimination between micronuclei containing a whole chromosome (centromere positive micronucleus) and an acentric chromosome fragment (centromere negative micronucleus).
The criteria for
selecting binucleated cells to score are the following:
Score binucleated cells with
Do not score:
In the absence of
cytochalasin B, mononucleated cells are analyzed for the presence of micronuclei.
In the presence of cytochalasin B, mononucleated cells are recommended to be
harvested at 24 hours post-PHA stimulation as there can be no doubt at this
time-point that MN within such a cell are a result of in vivo rather than
ex
vivo division. Binucleated cells are recommended to be harvested at 72 hours
post-PHA stimulation. Moreover, 24 hour post-PHA may be the right time to count
apoptotic/necrotic cells (Kirsch-Volders et al., 2001)
For the scoring of micronuclei the following criteria were adapted from Fenech et al., 1997:
Characteristics of the MN-test
Mononucleated cells à damage accumulated before cultivation of the cells
Binucleated cells à damage accumulated before cultivation of the cells + damage expressed during cultivation
Advantages/ Disadvantages
|
Advantages |
Disadvantages |
|
|
With/without cyto-B |
|
|
|
With cyto-B |
|
|
Confounding factors:
age
smoking?
References: